Abstract
Background: The quality of chimeric antigen receptor (CAR)-T cell products, including the expression of memory and exhaustion markers, has been shown to influence their long-term functionality. We previously demonstrated that piggyBac (PB) transposon-mediated CD19 CAR-T cells exhibit memory-rich phenotype that is characterized by a high proportion of CD45RA+/CCR7+ T cell fraction. To further investigate the favorable phenotype of PB-CD19 CAR-T cells, we generated PB-CD19 CAR-T cells from CD45RA+ and CD45RA− peripheral blood mononuclear cells (PBMCs) (RA+ CAR and RA− CAR, respectively), and compared their phenotype and antitumor function.
Methods: CD45RA+ and CD45RA− PBMCs were isolated by magnetic selection from whole PBMCs, then the CD19-CAR transgene was transduced into these cells using the PB transposon system, as described previously. Transduction efficiency of CD19 CAR transgene was determined 24 hours by flow cytometry after transduction. The phenotype of CD19 CAR-T was evaluated by flow cytometry on day 14. High throughput RNA sequencing was performed to see the T cell activation/exhaustion profile upon antigen stimulation. Sequential killing assays were performed by adding fresh tumor cells into CAR-T cells co-cultured with tumor cells every three days by restoring an effector target ratio of 1:1. To see the durable antitumor efficacy in vivo, we performed in vivo stress test, in which CAR T-cells dosage was lowered to the functional limits, so that these CAR-T cells should be maintained and expanded in vivo, to achieve the antitumor efficacy. We injected 5 x 10 5 of firefly luciferase-labeled CD19+ tumor cells (REH) into NSG mice via tail vein, then these mice were treated with 1 x 10 5 of CD19 RA+ CAR-T, RA− CAR-T, or control CAR-T cells, respectively, at day 6 after the tumor injection.
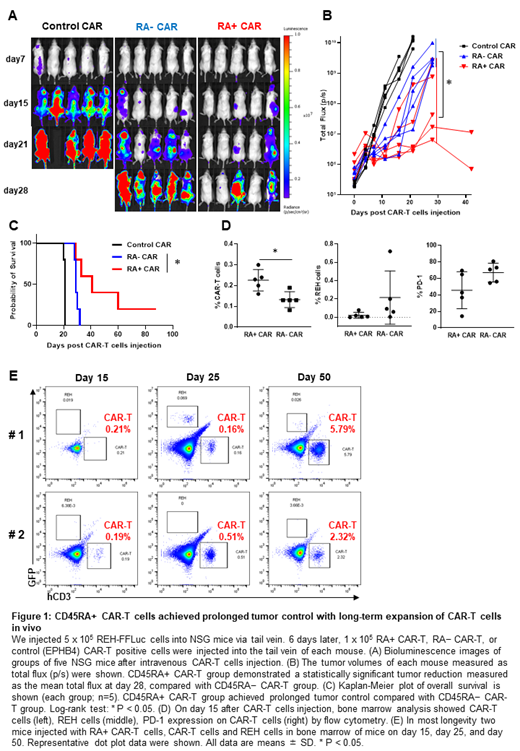
Results: RA+ CAR T cells demonstrated better transient transduction efficiency 24 h after transduction (RA+ CAR-T: 77.5 ± 9.8% vs RA− CAR-T: 39.7 ± 3.8%), and superior expansion capacity after 14 days of culture than RA− CAR-T cells (RA+ CAR-T: 32.5 ± 9.3-fold vs RA− CAR-T: 11.1 ± 5.4-fold). RA+ CAR-T cells exhibited dominant CD8 expression (RA+ CAR-T: 84.0 ± 3.4% vs RA− CAR-T: 34.1 ± 10.6%), less expression of exhaustion marker PD-1 (RA+ CAR-T: 3.1 ± 2.5% vs RA− CAR-T: 19.2 ± 6.4%) and T cell senescence marker CD57 (RA+ CAR-T: 6.8 ± 3.6% vs RA− CAR-T: 20.2 ± 6.9%), and enrichment of naïve/stem cell memory fraction (CAR+/CD45RA+CCR7+ fraction; RA+ CAR-T: 71.9 ± 9.7% vs RA− CAR-T: 8.0 ± 5.3%), which were associated with longevity of CAR-T cells. Transcriptome analysis revealed that RA+ CAR-T cells exhibited the enrichment of naïve/memory phenotype and less expression of canonical exhaustion markers, and these exhaustion profiles even maintained after the antigen stimulation. RA+ CAR-T cells demonstrated sustained killing activity even after multiple tumor rechallenges in vitro, without inducing exhaustion marker expression of PD-1. Although antigen stimulation could increase CAR expression, leading to tonic CAR signaling and exhaustion, in our study, the expression of CAR molecule on the cell surface following antigen stimulation in RA+ CAR was controlled at a relatively lower level that in RA− CAR-T cells. RA+ CAR-T cells achieved prolonged tumor control with expansion of CAR-T cells than RA− CAR-T cells in in vivo stress test (Fig.1A-C). On day15, bone marrow studies in RA+ CAR group exhibited abundant human CD3 positive T cells with less expression of PD-1, and relatively smaller amount of REH cells than RA− CAR group (Fig.1D). Furthermore, in two of long-lived mice in RA+ CAR group, human CD3 positive T cells were expanded even day 50 after treatment as confirmed by sequential bone marrow studies (Fig.1E), which indicated the antigen-induced proliferation and long-term functionality of RA+ CAR-T cells in vivo.
Conclusion: Our results suggest that PB-mediated RA+ CAR-T cells exhibit memory-rich phenotype and superior antitumor function, thereby indicating the usefulness of CD45RA+ PBMC as a starting material of PB-CAR-T cells.
Yagyu: AGC Inc.: Research Funding. Nagao: AGC Inc.: Current Employment. Kubota: AGC Inc.: Current Employment. Shimizu: AGC Inc.: Current Employment. Nakazawa: AGC Inc.: Research Funding; Toshiba Corporation: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal